Thousands Of Adverse Medical Events
Reported After Taking Coronavirus Vaccines Including
Death, Heart Attacks And Strokes Confirming Previous
Site ClaimsDecember 29. 2020

Pfizer
This is a follow up to several articles
I've written on this site about the dangers of the
coronavirus vaccines (see "RELATED ARTICLES" section
below). Since the vaccinations began over the past
3-weeks, there have been many reports of adverse medical
events. In December the public learned several people
died during the Pfizer trial. Two people died during the
AstraZeneca trial.
When the vaccines were rolled out,
people all over the world reported cases of anaphylactic
shock, which can turn fatal if medicine is not
immediately administered to counteract the effects of
said allergic reaction to the vaccines.
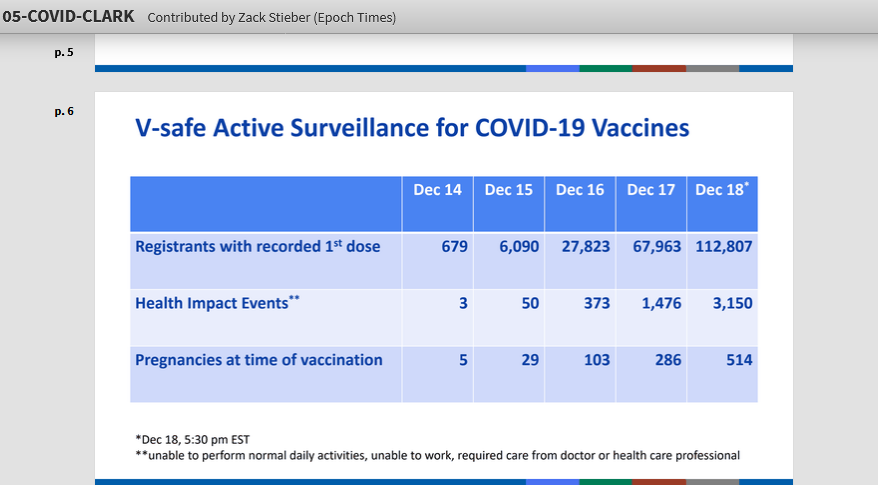
Report reveals 3,150 adverse "health impact
events"
There are 3,150 reports of adverse
medical events associated with the coronavirus vaccines.
One man in Israel even died of a heart attack after
receiving the coronavirus vaccine. Some people have also
developed Bells Palsy. The FDA issued a warning of
"severe adverse reactions" after taking the second dose
of the Pfizer vaccine.
The Egypt Independent reported, "The FDA
added that two participants died from heart attacks or
stroke, and the cause of death of two others is still
being determined." This confirms my previous statements
on Twitter.com from July 25, 2020 regarding the
then unreleased vaccines potential to cause heart
attacks, strokes and death, which has happened now
5-months later:


STORY SOURCE
FDA: Six people die during Pfizer/BioNTech’s
coronavirus vaccine trials
December 9, 2020 2:16 pm - Six people
died during trials of the coronavirus vaccine produced
by the American pharmaceutical company “Pfizer” and the
German “BioNTech” company, the US Food and Drug
Administration (FDA) said on Wednesday.
In a statement released by US channel
Al-Hurra, the FDA confirmed that among the deceased was
a participant who was obese and suffering from
arteriosclerosis and died three days after taking the
first dose of the vaccine.
The FDA added that two participants died
from heart attacks or stroke, and the cause of death of
two others is still being determined. “Of the six dead,
three are over the age of 55,” the FDA clarified.
According to Pfizer’s website, Phase Three of the
clinical trials included a total of 43,538 participants.
The FDA previously confirmed the
vaccine’s 95 percent efficacy rate, and supporting
documents have proven that the drug is safe and may be
used for emergency situations. The American
biotechnology company Moderna said in December that it
had submitted a request to the FDA to obtain a license
for emergency use of its vaccine, less than a year after
it began its clinical trials.
Moderna was the second company to
request emergency approval from the FDA, just two weeks
after Pfizer and BioNTech. If Moderna successfully
obtains permission from the FDA, it can begin giving
doses of its vaccine on December 21.
Moderna previously announced that its
vaccine against the coronavirus was 100 percent
effective against severe cases of the virus – a
significant leap in COVID-19 vaccine development.
Moderna joins Pfizer/BioNTech, Russsia’s Sputnik V, and
Oxford/AstraZeneca as the most successful coronavirus
vaccine manufacturers in the world as of now...
https://egyptindependent.com
FDA Says 2 Participants In Pfizer COVID Vaccine
Trial Have Died
Tuesday, Dec 08, 2020 - 13:30 -
With the FDA expected to grant emergency-use approval
for the Pfizer-BionTech COVID vaccine Thursday after
releasing a preliminary assessment of the trial data
that the panel will use to assess the drug earlier
today, the agency has admitted Tuesday that two
participants in the Phase 3 trials have died. One of
them was immunocompromised, according to the Jerusalem
Post, citing data released earlier.
The FDA is expected to release two
separate assessments of the trial data before a panel of
experts meets to review the data and either approve
Pfizer's request for emergency approval, or deny it.
This also comes after the FDA warned of a "severe averse
reaction" frequently seen in patients after taking the
second dose.
In the US, there has been at least one
other trial participant who reportedly died not long
after receiving the second dose. The participant in that
case was a priest in Philadelphia who participated in
the Moderna trial. In the UK, two patients were
seriously sickened during the trial of the AstraZeneca-Oxford
vaccine (though Oxford later said the illnesses had
nothing to do with the trial). While In Brazil,
authorities briefly halted a trial of Sinovac's
experimental COVID vaccine after a participant died...
https://www.zerohedge.com
FDA Confirms Pfizer Vaccine 95% Effective, Warns
Of 'Severe Adverse Reactions' After Dose 2
Tuesday, Dec 08, 2020 - 8:07 - Pfizer
and partner BioNTech’s COVID-19 vaccine meets
expectations on agency guidance and is enough to spur an
agency review, according to staff of the U.S. Food and
Drug Administration.
The finding is one of several
significant new results featured in the briefing
materials, which span 53 pages of data analyses from the
agency and from Pfizer. On Thursday, F.D.A.’s vaccine
advisory panel will discuss these materials in advance
of a vote on whether to recommend authorization...
https://www.zerohedge.com
Fairbanks clinician is third Alaskan with adverse
reaction to COVID-19 vaccine
Saturday, 19 December 2020 00:46 GMT -
ANCHORAGE, Alaska, Dec 18 (Reuters) - A Fairbanks
clinician suffered anaphylactic symptoms after being
given the Pfizer Inc coronavirus vaccine, a hospital
said on Friday, becoming the third Alaska health care
worker to suffer an adverse reaction to the new drug.
The clinician, whose name was not
released, started showing symptoms about 10 minutes
after being inoculated on Thursday, according to
Foundation Health Partners, operator of the Fairbanks
Memorial Hospital. The health care worker was treated in
the hospital's emergency room with epinephrine and
released about six hours later, Foundation Health
Partners said in a written statement.
Two health care workers in Juneau
suffered adverse reactions to the medication earlier
this week. One was briefly hospitalized in that city for
anaphylaxis after she was vaccinated on Tuesday. The
second had a milder reaction on Wednesday and was
treated at the hospital emergency room and released...
https://news.trust.org
UK issues anaphylaxis warning on Pfizer vaccine
after adverse reactions
December 9, 202010:31 PM - LONDON
(Reuters) - Britain’s medicine regulator said anyone
with a history of anaphylaxis to a medicine or food
should not get the Pfizer-BioNTech COVID-19 vaccine,
giving fuller guidance on an earlier allergy warning
about the shot.
Starting with the elderly and frontline
workers, Britain began mass vaccinating its population
on Tuesday, part of a global drive that poses one of the
biggest logistical challenges in peacetime history. The
Medicines and Healthcare Products Regulatory Agency (MHRA)
said there had been two reports of anaphylaxis and one
report of a possible allergic reaction since rollout
began.
“Any person with a history of
anaphylaxis to a vaccine, medicine or food should not
receive the Pfizer BioNTech vaccine,” MHRA Chief
Executive June Raine said in a statement. “Most people
will not get anaphylaxis and the benefits in protecting
people against COVID-19 outweigh the risks... You can be
completely confident that this vaccine has met the
MHRA’s robust standards of safety, quality and
effectiveness.”
Anaphylaxis is an overreaction of the
body’s immune system, which the National Health Service
describes as severe and sometimes life-threatening. The
fuller guidance, clarifying that the main risk was from
anaphylaxis specifically, was issued after consulting
experts on allergies. The MHRA had initially advised
anyone with a history of a “significant allergic
reaction” not to take the shot...
https://www.reuters.com
Oxford coronavirus vaccine results leave
regulators with 'dilemma', warns scientist
Tue, December 8, 2020, 10:25 AM EST -
The Oxford vaccine may not be as useful as first
thought, after published results showed that high
efficacy rates cannot be substantiated. At the end of
November, Oxford and AstraZeneca announced they had
efficacy rates of 90 per cent when people were given a
half dose, followed by a full dose several weeks later.
However when people received two full
doses, efficacy fell to just 62 per cent, far lower than
the mid-90s results shown by Pfizer and Moderna. The
results leave regulators with a "dilemma", scientists
have warned. The Oxford data is currently being studied
by the Medicines and Healthcare products Regulatory
Agency (MHRA) which will decide whether to approve the
jab. However, new data published in The Lancet on
Tuesday has led to fears that the more effective
half-dose regime will not be approved...
https://www.yahoo.com
Thousands Negatively Affected After Getting
Covid-19 Vaccine
https://www.theepochtimes.com
RELATED ARTICLES
China And New York Hospitals Using
Vitamin C To Aid In Treating Coronavirus Patients (Confirming Previous
Claims)
Reports Confirm Obesity And
Compromised Immune System Linked To Severity Of Coronavirus Infections
As Previously Stated On The Site
Scientists Confirm 8 Coronavirus
Strains Are Circling The Globe As Previously Stated On The Site (Covid-19)
New Scientific Study Confirms Coronavirus
Sufferers Having Difficulty Expelling CO2 As Previously Stated On The
Site
Coronavirus Infection Rate Skyrockets Among American Kids
With The Reopening Of Schools As Previously Predicted On The Site
Britain Death Toll From Coronavirus
Becomes The Highest In Europe Due To Prime Minister Boris Johnson's
Failed Herd Immunity Strategy
Theresa May And Boris Johnson
Under-Funding Of The NHS Left The Health Service Ill-Prepared To Counter
The Effects Of The Coronavirus (Covid-19)
Man In Hong Kong Contracts
Coronavirus For A Second Time Confirming Previous Site Claims (Video)
The Coronavirus Pandemic Has Sent The U.S. Economy
To Historically High Unemployment Levels (Video)
Coronavirus Vaccine
Makes Patient Seriously Ill With Transverse Myelitis Confirming Previous
Site Claims About Safety Issues
Coronavirus Vaccines In Trouble As Participant In
Research Trial Becomes Seriously Ill Confirming Previous Site Claims
Official Medical Studies State
Coronavirus Damages The Heart Confirming Previous Site Claims
Medical Associations And Researchers
Confirm Conjunctivitis Is A Symptom Of Coronavirus Confirming Previous
Site Claims
Overweight Teachers Have Been Dying
From Coronavirus Since School Reopening In America Confirming Previous
Site Claims
Boris Johnson And
Theresa May Failures In British Government Resulted In 25% Of Medical
Staff Contracting Coronavirus And Many Dying
New York Coronavirus Cases Came From
Europe
Johnson & Johnson Are The Second Pharma Giant To
Pause Coronavirus Vaccine Trials Due To A Participant Developing
Unexplained Illnesses Confirming Previous Site Claims
Another Person Contracts Coronavirus For The
Second Time And Worse Than Before Confirming Previous Site Claims
Pharmaceutical Giant Eli Lilly
Pauses Coronavirus Antibody Drug Due To Dangerous Side Effects
Confirming Previous Site Claims
President Trump Was Suffering From
Chest Pains When He Contracted Coronavirus Confirming Previous Claims
AstraZeneca Coronavirus Vaccine
Trial Participant Dies Confirming Previous Claims
The New York Times Interviews
Sufferers Of Covid-19 Brain Fog Who Are Exhibiting The Signs Of Dementia
Confirming Previous Site Claims
Cardiff University Lab Study
Demonstrates Mouthwash Kills Coronavirus Confirming Previous Site Claims
About Mouth Cleanliness
Face Mask Study Emphasizes Everyone Must Wear Them
In Order For Them To Work Against Coronavirus Confirming Previous Site
Claims
Britain Surpasses Over 1,500,000 Coronavirus Cases
Confirming Previous Claims
Professional Cyclist Contracts
Coronavirus Twice Confirming Previous Site Claims About Reinfection
Florida Gators Basketball Star Keyontae Johnson
Collapses On The Court And Is Placed In A Medically
Induced Coma Months After Contracting Coronavirus

