Remdesivir Ruled Ineffective In Scientific
Study By The World Health Organization Confirming Previous ClaimsOctober 16. 2020

Remdesivir
A study of 11,000 people conducted by the World
Health Organization (WHO), concluded the coronavirus "emergency use"
drug Remdesivir is ineffective and doesn't help people recover from
Covid-19. The study found no medical difference in patients who had
normal care or Remdesivir. The drug did not cut hospitalization time
or the mortality rate.
The report was released yesterday, just
days after pharmaceutical drug corporation Eli Lilly
suspended its antibody drug, developed in conjunction
with Remdesivir (Pharmaceutical Giant Eli Lilly
Pauses Coronavirus Antibody Drug Due To Dangerous Side Effects
Confirming Previous Site Claims).
U.S. President Donald Trump was given
Remdesivir while hospitalized 2-weeks ago with the
coronavirus (Covid-19). The president was also given
Regeneron's antibody drug, in tandem with Remdesivir,
while hospitalized at the Walter Reed medical hospital
in Bethesda, Maryland.
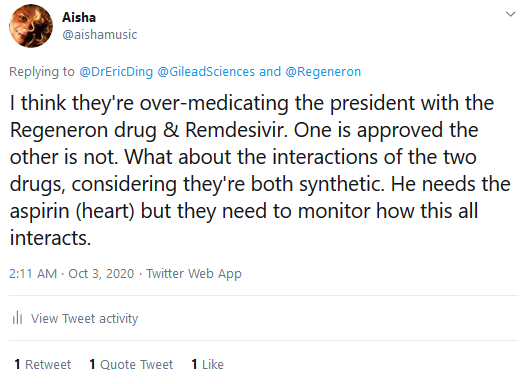
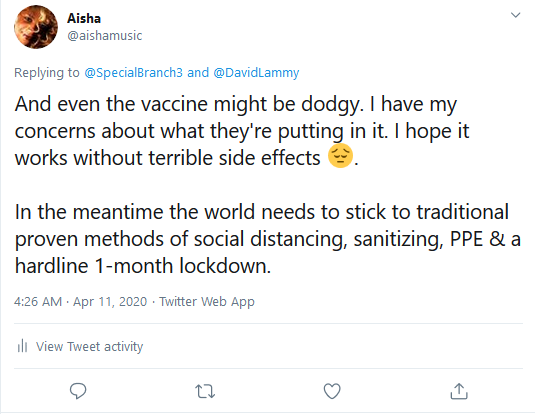
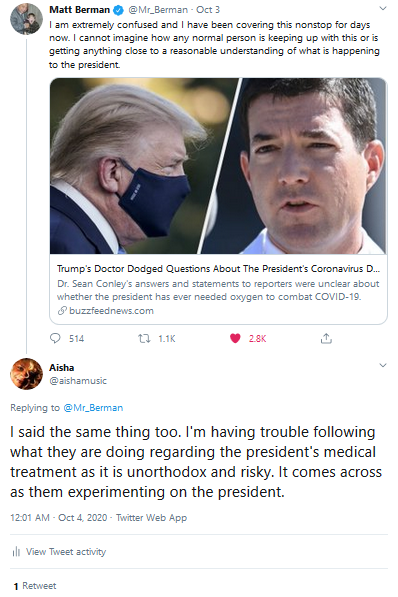
This confirms my previous tweets on
Twitter regarding Remdesivir where I questioned its
effectiveness (see below). Not to mention, the side
effects of the drug such as potential nausea, vomiting,
diarehea, rash, respiratory distress and liver damage
(and that's what's been declared so far).
Tweets from my Twitter page 2-weeks prior to the
announcement this week regarding safety concerns about the
monoclonal antibody class of drugs President Trump was given and the
ineffectiveness of Remdesivir:



STORY SOURCE
WHO study finds remdesivir didn't help COVID-19
patients
Yesterday- GENEVA (AP) ó A large study
led by the World Health Organization suggests that the
antiviral drug remdesivir did not help hospitalized
COVID-19 patients, in contrast to an earlier study that
made the medicine a standard of care in the United
States and many other countries.
The results announced Friday do not
negate the previous ones, and the WHO study was not as
rigorous as the earlier one led by the U.S. National
Institutes of Health. But they add to concerns about how
much value the pricey drug gives because none of the
studies have found it can improve survival.
The drug has not been approved for
COVID-19 in the U.S., but it was authorized for
emergency use after the previous study found it
shortened recovery time by five days on average. Itís
approved for use against COVID-19 in the United Kingdom
and Europe, and is among the treatments U.S. President
Donald Trump received when he was infected earlier this
month...
https://apnews.com

