Pharmaceutical Giant Eli Lilly Pauses Coronavirus Antibody
Treatment
Due To Dangerous Side Effects Confirming Previous Site ClaimsOctober 15. 2020

Eli Lilly
Pharmaceutical giant Eli Lilly has
paused a phase three trial of its monoclonal antibody
treatment for the coronavirus (Covid-19) due to "safety
concerns." CNBC reported this week, "The ACTIV-3 trial
is designed to test a monoclonal antibody developed by
Eli Lilly in combination with remdesivir, an antiviral
with emergency use authorization for the virus."

President Trump takes off his mask on the White House balcony
after being discharged from the hospital last week
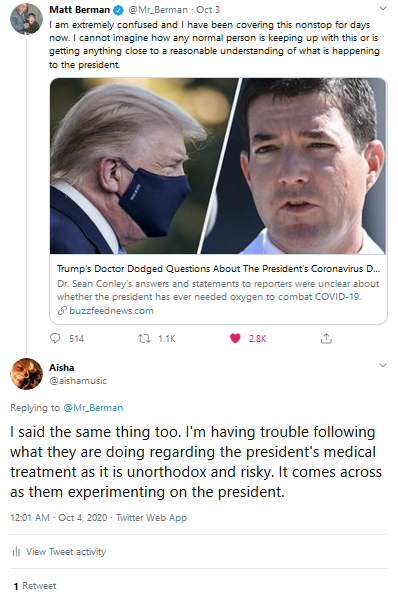
President Trump was taking Remdesivir last week,
after contracting the coronavirus (Covid-19). I repeatedly warned on
Twitter.com that is was risky administering an unproven drug to
anyone and this incident was the equivalent of experimenting on a
president:
Tweets from my Twitter page a week and a half prior to the
announcement this week regarding safety concerns about the
monoclonal antibody class of drugs President Trump was given:


STORY SOURCE
U.S. pauses Eli Lilly's trial of a coronavirus antibody
treatment over safety concerns
Published Tue, Oct 13 20202:36 PM EDT Updated Tue,
Oct 13 20205:01 PM EDT - Eli Lilly's phase three trial of its
ACTIV-3 monoclonal antibody treatment for the coronavirus has been
paused due to potential safety concerns.
The ACTIV-3 trial is designed to test a monoclonal
antibody developed by Eli Lilly in combination with remdesivir, an
antiviral with emergency use authorization for the virus. It's one
of several ongoing trials, as part of the National Institute of
Health's "Activ" program, designed to accelerate the development of
vaccine treatments in partnership with the pharmaceutical industry.
Eli Lilly's late-stage trial of its leading
monoclonal antibody treatment for the coronavirus has been paused by
U.S. health regulators over potential safety concerns, the company
confirmed to CNBC on Tuesday.
"Safety is of the utmost importance to Lilly. We are
aware that, out of an abundance of caution, the ACTIV-3 independent
data safety monitoring board (DSMB) has recommended a pause in
enrollment," spokeswoman Molly McCully told CNBC. "Lilly is
supportive of the decision by the independent DSMB to cautiously
ensure the safety of the patients participating in this study."
The company's shares closed down 2.9%. The news
comes less than 24 hours after Johnson & Johnson confirmed that its
late-stage coronavirus vaccine trial was paused after a participant
reported an "adverse event" the day before...
https://www.cnbc.com
RELATED ARTICLES
Johnson & Johnson Are The Second Pharma Giant To Pause Coronavirus
Vaccine Trials Due To A Participant Developing Unexplained Illnesses
Confirming Previous Site Claims
Another Person Contracts Coronavirus For The Second Time And Worse Than
Before Confirming Previous Site Claims
Coronavirus Vaccine Trial Makes More
Participants Sick With Mystery Illnesses Confirming Previous Site Claims
China And New York Hospitals Using
Vitamin C To Aid In Treating Coronavirus Patients (Confirming Previous
Claims)
Reports Confirm Obesity And
Compromised Immune System Linked To Severity Of Coronavirus Infections
As Previously Stated On The Site
Scientists Confirm 8 Coronavirus
Strains Are Circling The Globe As Previously Stated On The Site (Covid-19)
New Scientific Study Confirms Coronavirus
Sufferers Having Difficulty Expelling CO2 As Previously Stated On The
Site
Coronavirus Infection Rate Skyrockets Among American Kids
With The Reopening Of Schools As Previously Predicted On The Site
Britain Death Toll From Coronavirus
Becomes The Highest In Europe Due To Prime Minister Boris Johnson's
Failed Herd Immunity Strategy
Theresa May And Boris Johnson
Under-Funding Of The NHS Left The Health Service Ill-Prepared To Counter
The Effects Of The Coronavirus (Covid-19)
Man In Hong Kong Contracts
Coronavirus For A Second Time Confirming Previous Site Claims (Video)
The Coronavirus Pandemic Has Sent The U.S. Economy
To Historically High Unemployment Levels (Video)
Coronavirus Vaccine
Makes Patient Seriously Ill With Transverse Myelitis Confirming Previous
Site Claims About Safety Issues
Coronavirus Vaccines In Trouble As Participant In
Research Trial Becomes Seriously Ill Confirming Previous Site Claims
Official Medical Studies State
Coronavirus Damages The Heart Confirming Previous Site Claims
Medical Associations And Researchers
Confirm Conjunctivitis Is A Symptom Of Coronavirus Confirming Previous
Site Claims
Overweight Teachers Have Been Dying
From Coronavirus Since School Reopening In America Confirming Previous
Site Claims
Boris Johnson And
Theresa May Failures In British Government Resulted In 25% Of Medical
Staff Contracting Coronavirus And Many Dying
New York Coronavirus Cases Came From
Europe
Exclusives

